Noninvasive Neuromodulation:
A True Breakthrough in Alzheimer's Disease
Noninvasive Neuromodulation: A True Breakthrough in Alzheimer's Disease
therapy for Alzheimer’s Disease that works by enhancing neuroplasticity via neurostimulation of key
brain networks involved in memory.

THE TECHNOLOGY
A Bold New Approach with
Unprecedented Clinical Trial Results
A Bold New Approach with Unprecedented Clinical Trial Results
Sinaptica’s scientific co-founders have published unprecedented Phase 2 placebo-controlled results that show our closed-loop neuromodulation therapy slows Alzheimer’s disease progression by >80% at 6 months, as measured by four different gold-standard cognitive & functional clinical trial endpoints.
Based on this unprecedented data, our mission is to advance this breakthrough into large-scale pivotal studies and get it to desperate patients—and their caregivers—as soon as possible.
Playlist
PERSONALIZATION
Developing a Personalized Neuromodulation Therapy for Alzheimer's
THE TREATMENT
Closed-Loop Personalized
rTMS-EEG Neuromodulation
The Default Mode Network (DMN) is a key brain network involved in episodic memory and implicated in Alzheimer’s Disease progression. Our highly personalized approach is calibrated for each individual based on precise measurements from MRI, TMS-Evoked potentials (TEPs), and other data, allowing us to individualize stimulation to achieve optimal response, safely.
Each patient’s brain is unique, and responds differently to neuromodulation. Our therapy uses neuronavigation to precisely and repeatably optimize electrical stimulation of the precuneus, which is the central hub of the DMN.
THE RESULTS
Unprecedented
>80% disease slowing on four gold-standard
cognitive & functional endpoints
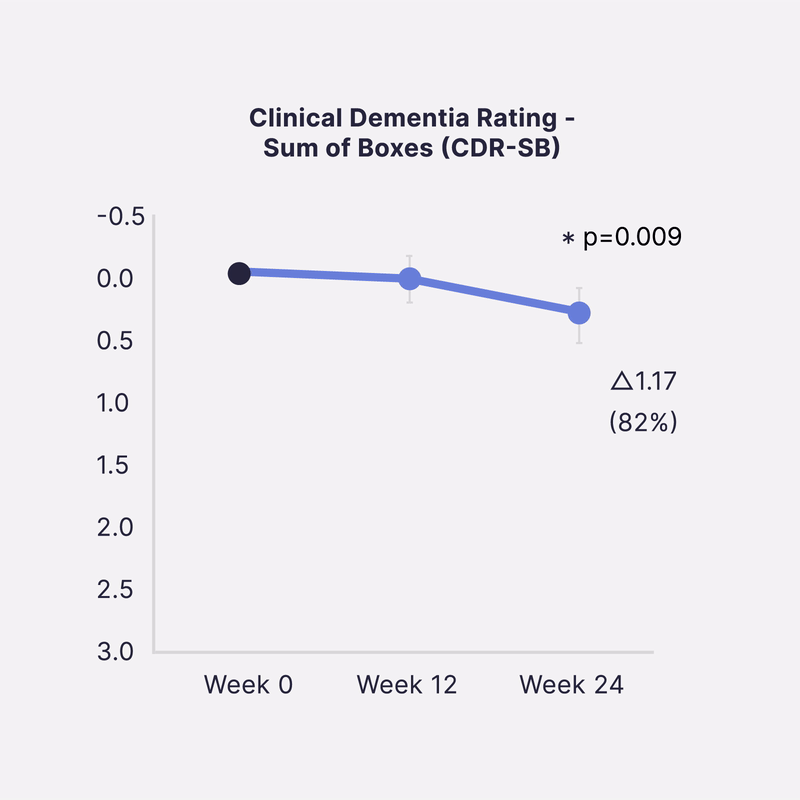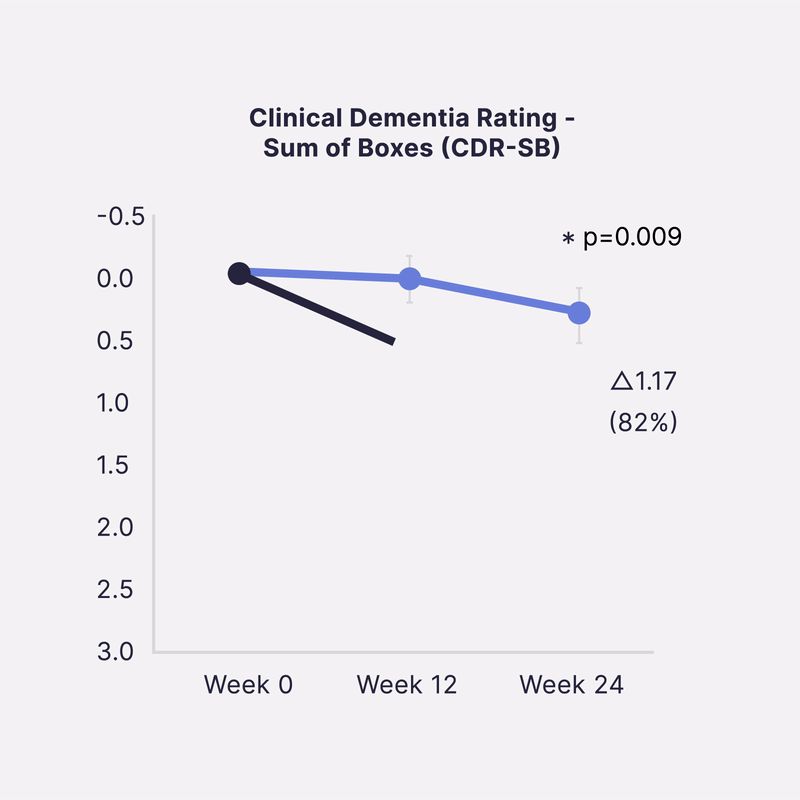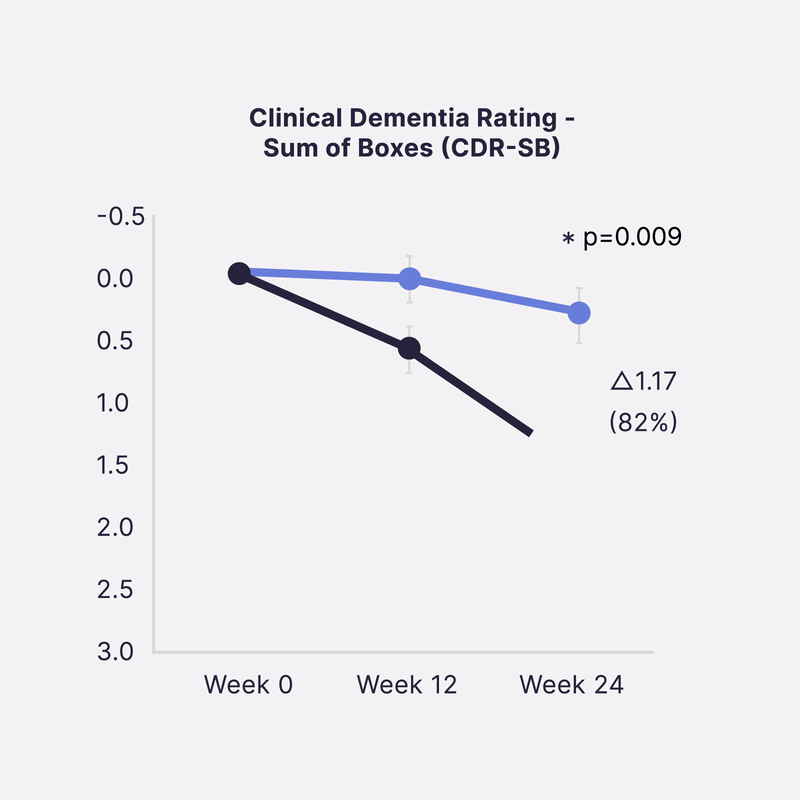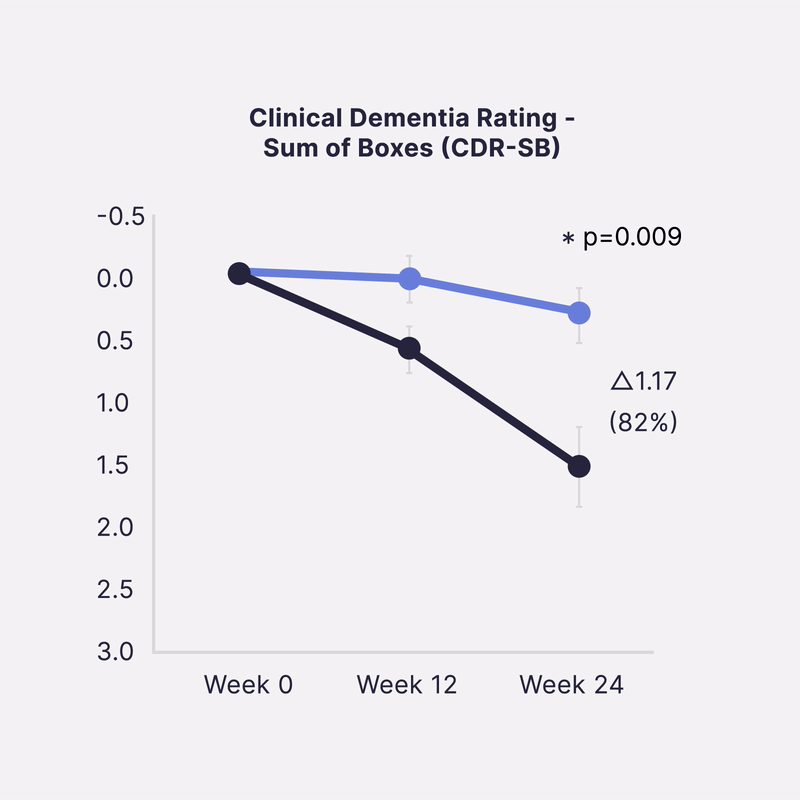
In a 50-patient randomized double-blind placebo controlled study, Sinaptica’s personalized rTMS-EEG neuromodulation was shown to profoundly preserve cognition and function after 6 months, compared to placebo. These results were published in the peer-reviewed Oxford University Press journal, Brain.
SCIENTIFIC CO-FOUNDERS
World-class Scientific Team

Professor of Physiology, University of Ferrara.
Director, Brain Stimulation Laboratory, Santa Lucia Foundation.



Associate Professor of Neurology & Radiology, Harvard Medical School. Director, Precision Neuroscience and Neuromodulation Program at Massachusetts General Hospital.






